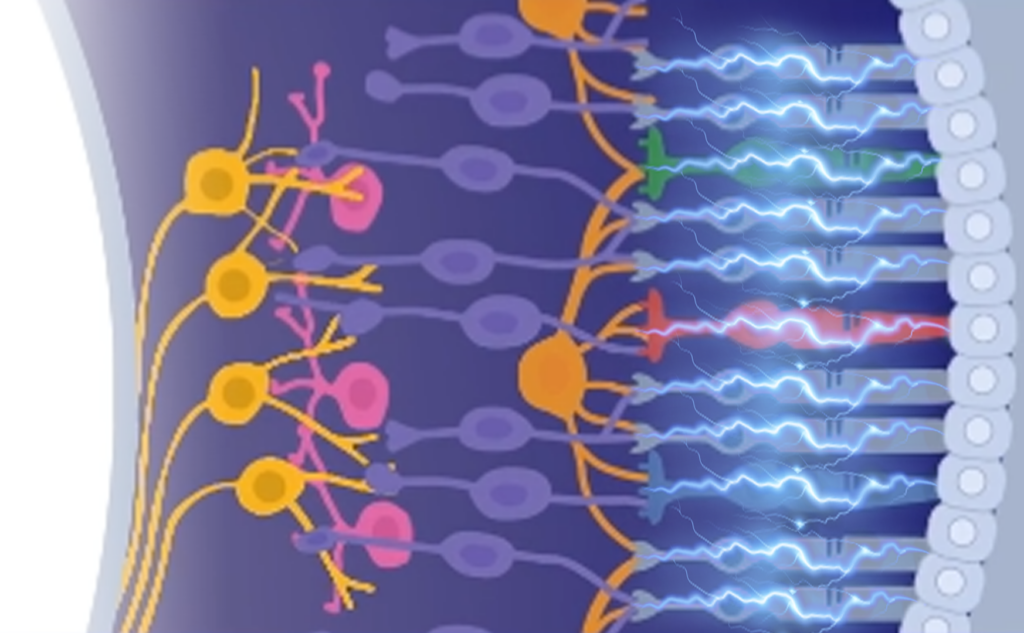
Leverage our experience supporting ophthalmic trial design and data reading
We have over 35 years of experience working with pharmaceutical, biotech, and CRO companies, supporting more than 100 clinical and preclinical trials. Our trial services include study setup assistance, training, and trial data reading. These services enable sites to gather consistent, quality data across multiple locations worldwide.
Trial services for preclinical research
We have a rich history of being involved in all phases of preclinical research. Our preclinical Celeris system is currently being used to study genetics in countless labs worldwide.
Trial services for gene therapy
Our DiagnosysFST was used as an endpoint for LUXTURNA, the first FDA approved gene therapy and continues to be an endpoint for the efficacy of this treatment.
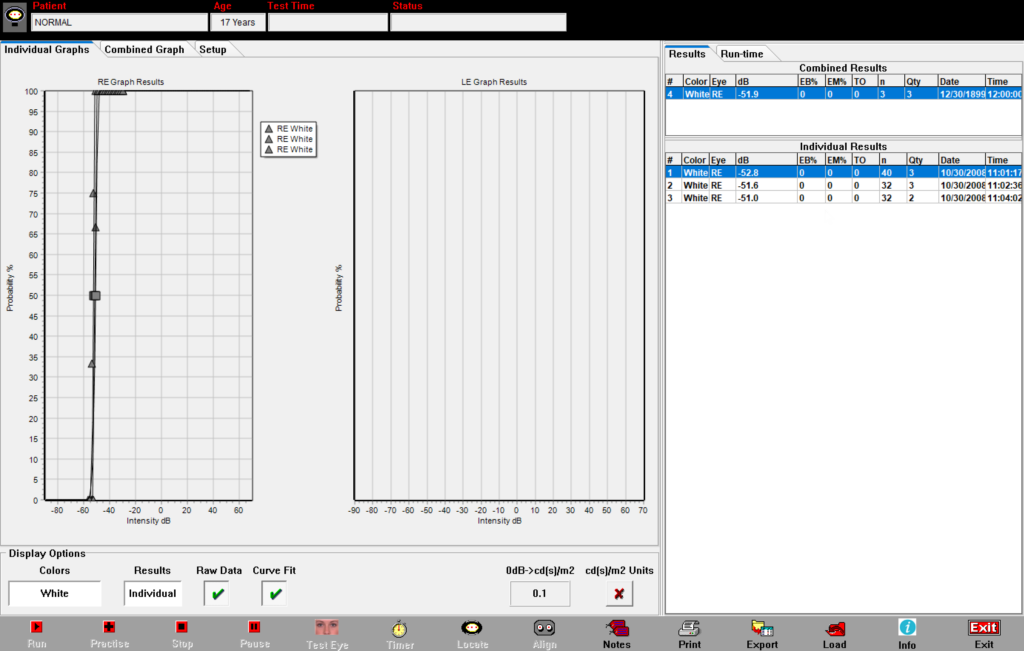
Quantify luminance thresholds for low vision patients with DiagnosysFST
Electroretinography (ERG) can phenotype inherited retinal diseases (IRDs), but when the ERG goes flat, FST quantifies residual function. DiagnosysFST, a Full-field Stimulus Test, quantifies the lowest levels of visual perception. This psychophysical method involves a 2-button response box, wherein patients affirmatively respond Yes or No to seeing a flash. This method continues to evolve as treatments advance.
EER: Electrically Evoked Response
Use EER on patients with no light perception to screen candidates for regenerative therapies. An EER electrically stimulates the visual system. This method of stimulation bypasses the photoreceptors, directly stimulating post-photoreceptor pathways. Diagnosys’s patented EER stimulates and measures an electrical response.
This method is not currently FDA approved.