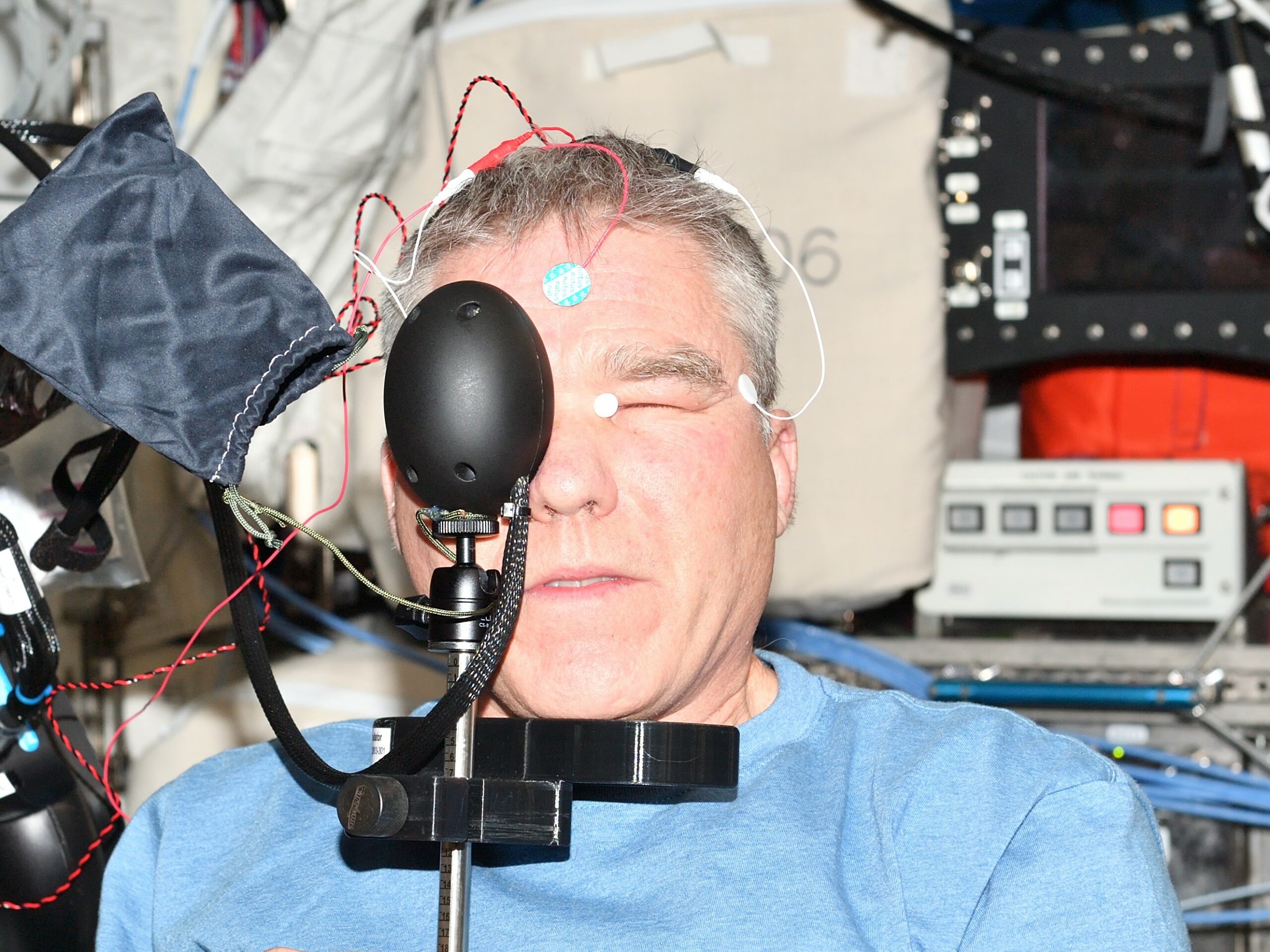
Diagnosys E3 Ophthalmic Electrophysiology System begins its use on the International Space Station. Research on changes to the eye and brain during long-duration spaceflight now uses the Diagnosys E3 system to measure retinal function.
Lowell, MA – Diagnosys LLC announced that its E3 system with Envoy and ColorBurst stimulators has been successfully installed at the International Space Station (ISS) and are now fully operational and in use. Diagnosys is a privately-held ophthalmic electrophysiology and psychophysiology technologies company focused on diagnostic solutions that enable researchers and clinicians to improve patient care.
NASA launched the E3 system with Envoy and ColorBurst to the ISS aboard a Northrop Grumman Antares rocket, from its Wallops Flight Facility in Wallops Island, Virginia on August 10th 2021. The system was placed into service in early 2022 and was first used to test an ISS crew member as part of a research study in April 2023, marking the first time in history that a human electroretinography (ERG) test has been conducted in space.
According to NASA, almost 70% of astronauts’ eyes demonstrate early signs of structural change during long duration spaceflight, for example swelling at the back of the eye. The long-term health consequences are unknown, but are an area of active investigation at NASA. Weightlessness causes blood and presumably cerebrospinal fluid to shift toward the head. This fluid shift is believed to be the underlying cause of the eye and brain structural changes in crew members on long duration space missions.
“Our team is very proud of the collaboration with NASA to have our E3 system be a part of this program,” said Jeffrey Farmer, Diagnosys CEO. “The Diagnosys E3 system is being used to conduct PhNR and PERG tests, which are also used in clinics to test glaucoma patients. The tests measure the function of the retina as it’s stimulated with a light source and in particular measure key functional attributes of the retinal ganglion cells (RGCs).”
The Diagnosys E3 system represents the next-generation ERG technology as it provides advanced analytics for both the PhNR and PERG tests to increase repeatability between exams, decrease test time and improve patient comfort during the test by using DTL electrodes. The Diagnosys system in use by NASA is the same system used by clinical customers with some slight ergonomic adjustments for the PERG test to be completed in a low gravity environment.
“Utilizing the Diagnosys E3 allows NASA to gain greater understanding of Spaceflight Associated Neuro-ocular Syndrome (SANS), a condition astronauts commonly experience as a result of space flights and can cause optic disc edema,” said Alex Huang, MD, PhD, Associate Professor, Department of Ophthalmology, Shiley Eye Institute, University of California, San Diego.
“One of our goals in utilizing advanced analytical techniques developed by Diagnosys and used to analyze the ERG tests conducted on astronauts, is improving our understanding of RGC function and intertest reliability than has been possible in the past.” said Rustum Karanjia, MD, PhD, Assistant Professor, Department of Ophthalmology, University of Ottawa Eye Institute.